As some of you will recall from Blog #1, one of the basic concepts of Quantum Mechanics is that electrons can behave like both waves and particles.There is a classic experiment that demonstrates this wave-particle duality, which I will get to in a minute. I don't believe that an electron's wave-particle duality has ever been modeled mechanically. There might be a good reason for that - or maybe it's just that nobody has tried. It turns out I have a concept that might get us there - or at least part of the way there.Wave-Particle DualityI have pulled some text explaining the history of this concept from a book called, Programming the Universe: A Quantum Computer Scientist takes On the Cosmos, by Seth Lloyd, First Vintage Books, 2007.Lloyd starts here with quotation marks belonging to Danish physicist Niels Bohr, who (I guess) originally coined the phrase/concept "wave-particle duality"."Wave-particle duality" refers to the fact that things we normally think of as waves, like light and sound, are actually made up of particles, or quanta (Quantum is the Latin word for "how much"). Particles of light are called photons ("-on" being the usual suffix denoting a particle); particles of sound are called phonons.The notion that waves are actually made of particles is very old. That sound is made of waves has been known since Pythagoras, but the ancient Greeks thought light was composed of particles and argued over whether these particles came from the eye or from the object viewed. Newton proposed a "corpuscular theory" of light in terms of particles.But Newton's own experiments with prisms were more easily explained if light was made of waves, and the wave theory of light dominated from the seventeenth century until the end of the nineteenth, culminating in Maxwell's equations, which explained all known electromagnetic phenomena in terms of waves of light.There was a problem thinking of light only in terms of waves ... At the end of the nineteenth century, Max Planck ... was able to resolve this problem by assuming that light was made out of particles whose energy was proportional to the frequency of the wave. Planck called these particles photons. Each photon carried a small amount of energy - a quantum. Planck found that ... the energy of each of these particles (measured in joules) was equal to 6.63 x 10^-34 times the waves frequency per second. Planck's constant ... is so ubiquitous in physics that it has been given its own special symbol, h.Planck's Constant using something that looks like an "h" because there is no "h" in "Planck's Constant"
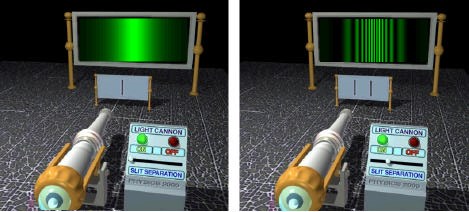
~Rory AndrewsJust as every wave is made up of particles, every particle - an electron, an atom, a pebble - has a wave associated with it. The wave is associated with the position of the particle: the particle is more likely to be found in places where the wave is big. The distance between the peaks of the wave is related to the particle's speed: the smaller the distance from peak to peak, the faster the particle is going. Finally, the waves frequency is proportional to the the energy of the particle. In fact, the particles energy is exactly equal to the frequency times Planck's constant.The Double-Slit ExperimentThe double-slit experiment demonstrates the wave nature of particles. Waves superpose, or interfere, with each other. When the peaks of two waves meet, they form a very large wave. If the peak of one wave meets the trough of another, they will cancel each other out.Light waves will combine with each other in the same way. If you shine a beam of light at at a screen with two slits in it and look at the pattern the light makes on the wall beyond the screen, you will see alternating bands of light and dark. This is called an "interference pattern." The light waves, moving light water waves through pilings, go through both slits at once; each wave thus splits in two and then combines on the wall. The bands of light occur in places where the peaks and troughs of the wave from one slit coincide with the peaks and troughs of the wave of the other wave and reinforce them, a phenomenon called "positive interference." The bands of dark occur where the peaks of the wave from one slit coincide with the troughs of the other wave and the two waves cancel each other out, a phenomenon known as "negative interference."If you cover one of the slits, the interference pattern goes away, because there is no wave to interfere with the wave that goes through the remaining slit. Interference, crucially, requires the wave to go through both slits at once.

- Every particle has a wave associated with it.
- Waves superpose, or interfere, with each other.
- Now shoot a single electron at the screen. The interference pattern is still there, but now it simply governs the probability for where the electron arrives on the photographic plate: it preferentially lands where the band of spots lies.
- The [twin-slit] experiment reveals that the particle goes through both slits at once.
I promised, earlier on, that I had a mechanical concept to demonstrate this concept, and I do. But I think I have given us enough to think about for the moment. I will the save 'big idea' until the next blog.
In the meantime, let me leave you (tease you) with this picture of something that is collecting dust in my basement. It is part of my proposed solution.

Thank you for joining me for Blog #2. Stay tuned and keep your toolboxes at ready, fellow (quantum) Mechanics. Blog #3 is coming.
P.S. I would like to thank Rory (Rien-de-Photo-Captions)(Click Bait) Andrews for helping me choose some of the graphics for today's blog. I really appreciate your good humour and spirit-of-collaboration, Rory.







0
Log In or Sign Up to add a comment.- 1
arrow-eseek-eNo items to displayFacebook Comments